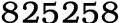|
Ammonium acetate is a metallic salt that takes the form of a white crystalline solid at room temperature, and has both weak acid and weak base properties, making it useful in a variety of industries. It is used in the manufacture of synthetic polymers, such as in rayon fiber production and vinyl plastic production, and as a metallic mordant or fixative compound in the dyeing and printing of textiles. In the food industry, ammonium acetate is also used to preserve meat, and high-grade versions of the compound are used to manufacture common pharmaceuticals such as insulin and penicillin. Due to its diversity of uses, it is available commercially as a crystalline powder or in a solution of water at concentrations of usually 50% and 57.5%. One of the primary hazards that ammonium acetate use poses involves where it is employed as a deicing agent for road surfaces or aircraft. While it is both inexpensive in raw form and considered biodegradable, it is a form of nitrogen salt that can easily leach into soil and groundwater sources, adversely affecting vegetation. The health risks of the compound are considered fairly mild, however, and include mild irritation of the skin and eyes upon contact, as well as causing limited respiratory or gastrointestinal irritation if inhaled or ingested. Another common use for dissolved ammonium acetate is as a buffer solution since it normally has a pH in the range of 7, which is right in the middle of the pH range overall, with levels below 7 being acidic and levels from above 7 up to 14 being considered basic compounds. This mirrors the pH of water, which is also rated a 7, and both water and ammonium acetate are compounds that are used to buffer, or maintain, the pH of other chemicals at their current levels even when further acidic or basic materials are added. Buffering agents are important in many industries such as food processing, industrial detergent manufacturing, and pharmaceutical production. Ammonium acetate is volatile at low pressures. Because of this it has been used to replace cell buffers with non-volatile salts, in preparing samples for mass spectrometry.It is also popular as a buffer for mobile phases for HPLC with ELSD detection for this reason. Other volatile salts that have been used for this include ammonium formate. In rubber latex production, ammonium acetate is used as a gelling or foaming agent, which serves to trap air bubbles within the latex while it is being set. This makes it useful primarily in the manufacture of foam rubber compounds as opposed to more solid types of industrial-strength rubber. Foam rubber is widely used in packaging and insulation, as well as for decorative hats and costumes, or as durable sponge-like sports equipment and cushioning materials used in couches, beds, and chairs.
Related Articles -
Ammonium acetate, 631-61-8,
|